Abstract
Introduction:
Recently we described a subgroup of pediatric patients with B cell precursor acute lymphoblastic leukemia (BCP ALL) with switching from B to monocytic lineage in early phase of the therapy (Slamova et al., 2014). In a limited cohort of patients with switching ALL (swALL), we observed inferior response to treatment with discrepancy of minimal residual disease level (MRD) assessed by flow cytometry (FC) and quantitative polymerase chain reaction (qPCR) of Immunoglobulin-T cell receptor (Ig-TCR) rearrangements. In current Berlin-Frankfurt-Münster (BFM) treatment protocols, FC MRD value at day 15 (d15) and PCR MRD value at day 33 (d33) and week 12 (w12) are used for stratification. Using an extended cohort of patients with available RNA sequencing data (cohort mainly focused on B other cases or swALLs) we aimed to answer following questions:
What is the genetic background of swALL? What is the frequency among swALLs of the recently described DUX4 rearranged subgroup?
How do B cell oriented FC and PCR MRD correlate in standard protocol timepoints, i.e. day 8 (d8) (peripheral blood, PB) d15, d33 and w12 (bone marrow, BM) of treatment?
What is the characteristic MRD response to treatment in swALL?
Results:
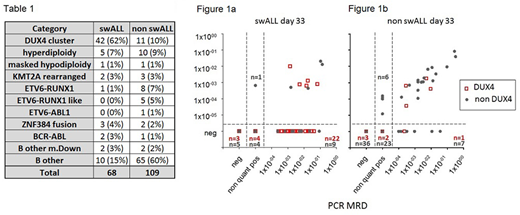
We performed RNA sequencing in 177 patients (median age 6.1 years, range 0-18) treated by several treatment protocols (ALL BFM 95 n=5, ALL IC BFM 2002 n=14, ALL AIEOP BFM 2000 n=17, ALL AIEOP BFM 2009 n=135, Interfant n=3, ALL IC/Interfant n=2, EsPhALL n=1). In 68 patients we observed switching phenomenon by appearance of B/monocytoid population coexpressing B lineage (CD19, CD34) and monocytic lineage (CD33, CD14) markers (median 0.98%, range 0.032-38%). In non swALLs median of this population was 0.059% (range 0.0025-1.1%) and the cells did not form a clear cluster. According to RNAseq data, majority of swALL patients (n=42/68) belong to DUX4 subgroup (chi square p< 0.00001). The distribution into other molecular genetic subtypes is summarized in table 1.
Correlation coefficient (Spearman) of all included samples with both available values (n=552) was 0.82 (p<0.0001), Concordance in categorization of positivity and negativity with cut-off 1e-4 was 85%. We observed worse correlation between FC and PCR MRD in patients with swALL (d8 R=0.58, d15 R=0.6, d33 R=0.36, w12 n.s.) compared to non swALL (d8 R=0.83, d15 R=0.91, d33 R=0.69, w12 R=0.37). However, concordance in swALL in categorization of positivity and negativity with cut-off 1e-4 was still ≥80% in each analyzed timepoint apart from d33 with concordance only 44% showing significant discrepancy of both methods (Figure 1a). On the contrary, concordance in non swALL was ≥87% for each analyzed timepoint (d33 with concordance 87% shown in Figure 1b). Poor correlation between B-cell oriented FC MRD and PCR MRD at d33 was also obvious when analyzed DUX4 subgroup separately (R=0.31 (p=0.04), concordance 45%).
We observed significantly higher MRD in swALLs compared to non swALLs at all analyzed timepoints: d8 (p=0.0021), d15 (p=0.0088, d33 (p<0.0001) and w12 (p=0.008). Higher MRD levels were also found in DUX4 patients when compared to non DUX4 (all timepoints p<0.05). Interestingly, when compared swALL and non swALLs pts in DUX4 subgroup only, the DUX4 swALLs are those with poorer treatment response (all timepoints p<0.05). With respect to protocolar cut-off values, FC MRD at d15 was above 10% in 18/67 swALL patients (in 13/52 DUX4 patients), d33 PCR MRD was above 0.1% in 33/57 swALLs (24/44 DUX4 pts) and at w12 PCR MRD was above 0.01% in 12/55 swALLs (10/44 DUX4 pts).
Conclusions:
DUX4 subgroup is the most prevalent genetic subtype among swALLs. SwALLs and/or DUX4 subgroup have poorer treatment response at d15, d33 and w12. However, it remains to be elucidated whether poor initial treatment response is eventually reflected in treatment outcome. In majority of swALL patients B cell phenotype of blasts is preserved at day 15 enabling correct classification. Prominent discrepancy between FC and PCR MRD is present especially at d33 and development of different FC MRD strategies focused on monocytic compartment is needed.
Supported by Ministry of Health of the Czech Republic, grant nr. 15-28525A and NV18-03-00343; Czech Science Foundation nr.P302/12/G101, UNCE204012
Brüggemann:Affimed: Research Funding; Regeneron: Research Funding; Amgen: Consultancy, Research Funding, Speakers Bureau; Roche: Speakers Bureau; Pfizer: Speakers Bureau; Incyte: Consultancy; PRMA: Consultancy. Ritgen:abbvie: Research Funding; Roche: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal